Lithium Aluminum Hydride Market
Lithium Aluminum Hydride Market Analysis, By Purity, By Preparation Method, By End-Use Industry, and By Region - Market Insights 2025 to 2035
Analysis of the Lithium Aluminum Hydride Market Covering 30+ Countries Including Analysis of the US, Canada, UK, Germany, France, Nordics, GCC countries, Japan, Korea, and many more
Lithium Aluminum Hydride Market Outlook (2025 to 2035)
The global lithium aluminum hydride market is expected to be valued at USD 190.1 million by 2025, according to Fact.MR analysis indicates that the industry is expected to grow at a CAGR of 5.2% and reach USD 313.3 million by 2035.
In 2024, the industry experienced a moderate expansion, reaching an estimated value of around USD 180.7 million, up from USD 171.7 million in 2023. The year was marked by incremental but targeted demand growth across the electric vehicle (EV) and hydrogen fuel cell ecosystems, especially in North America and Japan. Notably, small- to mid-scale automotive OEMs and component suppliers in the USA have begun scaling up R&D and pilot-phase applications using lithium aluminum hydride (LAH) as a hydrogen storage and transfer agent.
A granular observation reveals that downstream demand from battery-grade applications grew cautiously amid concerns about costs. In contrast, procurement by academic and industrial laboratories saw slight increases due to government-funded clean energy research programs. However, supply remained tight, with procurement cycles stretching longer due to regulatory constraints and quality control requirements related to the compound’s pyrophoric nature.
Looking ahead to 2025 and beyond, the LAH industry is poised to grow steadily, supported by emerging hydrogen economy frameworks, particularly in East Asia and the USA. The ongoing technological integration of LAH in portable fuel systems and next-generation propulsion technology could unlock further commercial applications. Strategic partnerships, particularly between specialty chemical firms and clean-tech startups, are expected to define the next phase of expansion.
Key Metrics
| Metrics | Values |
|---|---|
| Industry Size (2025E) | USD 190.1 million |
| Industry Value (2035F) | USD 313.3 million |
| Value-based CAGR (2025 to 2035) | 5.2% |
Don't Need a Global Report?
save 40%! on Country & Region specific reports
Fact.MR Survey on Lithium Aluminum Hydride Industry
Fact.MR Stakeholder Survey Outcomes: Forecasts by Stakeholder Opinion
(Q1 2025 Survey, n=500 evenly divided between chemical manufacturers, advanced materials distributors, battery researchers, automotive OEMs, and fuel cell tech developers from USA, Western Europe, China, and Japan)
Most Important Goals of Stakeholders
- Standards for Purity & Reactivity: 84% of international respondents identified producing ultra-high purity (≥98%) as highly important for applications in batteries and fuel cells.
- Safe Handling & Storage: 73% urged improved packaging and containment to address LAH's pyrophoricity and moisture sensitivity.
Regional Variance:
- USA: 67% prioritized supply chain redundancy owing to regulatory uncertainty surrounding hazardous materials.
- Western Europe: 81% stressed environmental compliance and low-emission synthesis routes.
- China: 72% prioritized cost-effective scaling of LAH for wider application in hydrogen storage.
- Japan: 64% prioritized integration compatibility with next-generation solid-state battery systems.
Technology Adoption Trends
Divergent Approaches:
- USA: 61% of OEMs and researchers invested in LAH-modified hydrogen fuel systems for fleet applications.
- Western Europe: 56% developed LAH in test energy-dense portable power systems for military and aerospace.
- China: 44% of applications involved LAH in modular reformer units, supported by public-private investment in hydrogen infrastructure.
- Japan: A mere 28% embraced next-gen LAH technologies, attributing this to "doubtful performance benefits" in humid environments.
ROI Perspectives:
- USA/EU: 69% perceived LAH-based systems as a worthwhile R&D investment, while Japan was cautious, with only 31% viewing them as a clear ROI.
Form Preferences of Materials
Consensus:
- Powdered LAH: Preferred by 70% of stakeholders for its reactivity, particularly in hydrogen generation.
Regional Trends:
- Western Europe: 51% opted for granulated forms with surface coating to reduce handling risk.
- Japan/China: 47% preferred encapsulated versions (e.g., polymer-coated) for precision uses.
- USA: 66% remained with the traditional powder form but asked for innovation in moisture-barrier packaging.
Price Sensitivity
Cost Pressure:
- 87% mentioned that increasing raw material costs, particularly for aluminum and lithium precursors, were affecting project economics.
Regional Sensitivities:
- USA & EU: 59% were willing to pay a premium of 10-15% for safer and higher-purity LAH.
- China: 74% wanted pricing less than USD 700/kg to facilitate wider deployment.
- Japan: 42% required performance-based pricing arrangements, particularly for niche pilot-scale uses.
Pain Points in the Value Chain
Manufacturers:
- USA: 58% experienced a shortage of workforce in handling and scaling up pyrophoric materials.
- EU: 52% identified REACH-compliance roadblocks in postponing product introductions.
- China: 61% experienced unreliable access to lithium hydroxide feedstocks.
- Japan: 49% reported R&D partnerships with local universities as lacking in leading breakthrough innovation.
Distributors:
- USA: 65% identified hazmat transportation regulations as major logistic challenges.
- EU: 48% identified low margins due to heavy regulatory expenses.
- China/Japan: 60% encountered delays in packaging due to changing safety certification requirements.
End-Users:
- EU: 44% experienced LAH products as insufficiently documented for performance.
- Japan: 54% mentioned "long turnaround times" for batches of custom-formulated LAH.
Priorities for Future Investment
Global Alignment:
- Seventy-two percent of LAH producers are looking to invest in automated, sealed packaging systems to decrease handling risk.
Regional Divergence:
- USA: 64% in integrated LAH-fuel cell modules for the defense and logistics sectors.
- EU: 59% in low-emission LAH synthesis based on recycled aluminum.
- China: 66% in bulk LAH production through domestic lithium supply chains.
- Japan: 46% in nano-engineered LAH for next-generation energy storage R&D.
Regulatory Impact
- USA: 69% of respondents indicated that OSHA and EPA regulations are becoming increasingly complex for managing pyrophoric compounds.
- EU: 83% reported that environmental regulations (e.g., CLP, REACH) compel more environmentally friendly LAH production.
- China: 41% indicated that export-oriented safety regulations influence bulk shipment models.
- Japan: Only 29% believed local legislation had a significant impact on procurement, referencing the negligible policy emphasis on Local Area Health (LAH).
Conclusion: Consensus vs. Regional Nuance
High Consensus:
- Concerns regarding purity, safety, and cost are at the forefront globally.
Key Variances:
- USA: Encouraged by performance-based pilot initiatives and hydrogen economy enthusiasm.
- EU: Dominant in sustainable LAH synthesis and regulatory complexity.
- China: Price-conscious scaling and local supply chain focus.
- Japan: Proceeding cautiously with focused research and modest mass aspirations.
Strategic Insight:
A region-by-region approach is needed- ultra-pure variants in Japan/EU, bulk production in China, and R&D kits packaged in the USA are on the path to unlocking industry potential.
Government Regulations on Lithium Aluminum Hydride Industry
| Country | Policy & Regulatory Impact |
|---|---|
| USA |
|
| European Union |
|
| China |
|
| Japan |
|
| South Korea |
|
| India |
|
More Insights, Lesser Cost (-50% off)
Insights on import/export production,
pricing analysis, and more – Only @ Fact.MR
Market Analysis
The LAH industry is expected to grow steadily, with increasing demand in pharmaceuticals, specialty chemicals, and cutting-edge energy storage solutions. Tough safety regulations and supply chain pressure, particularly from export-restrictive countries like China, are redefining sourcing and production strategies. Companies with robust regulatory compliance strengths and diversified supply chains will benefit, while those depending on single-source imports will experience serious setbacks.
Top 3 Strategic Imperatives for Stakeholders
Diversify Raw Material Sourcing and Enhance Supply Security
Executives need to actively invest in creating alternative sources of lithium and aluminum compounds outside China to offset geopolitical and regulatory risks. Strategic alliances with local suppliers and localized stockpiling can insulate against export controls and price volatility.
Align Product Development with Regulatory and End-Use Trends
Focus on formulation and product development breakthroughs that address changing safety, handling, and purity requirements-particularly for pharma and fuel cell use. R&D groups must work closely with regulators and final-user industries to predict changes and minimize time-to-industry.
Extend Global Reach through Strategic Partnerships and M&A
Investigate cross-border joint ventures or buy niche chemical firms with LAH handling capacities in high-growth areas such as North America and East Asia. Regional production facilities and in-house custom synthesis investments will improve agility and regulatory compliance.
Top 3 Risks Stakeholders Should Monitor
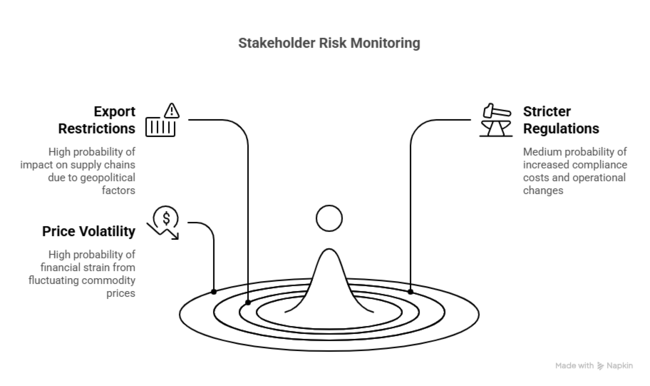
| Risk | Probability/Impact |
|---|---|
| Export Restrictions from China on Key Precursors: China is a dominant producer of lithium and aluminum intermediates. | High |
| Stricter Global Safety Regulations for Handling Reactive Compounds: Regulatory agencies (e.g., OSHA, REACH, EPA) are tightening rules on handling pyrophoric and moisture-sensitive chemicals like LAH. | Medium |
| Volatility in Lithium and Aluminum Commodity Prices: Fluctuating prices due to geopolitical tensions, mining restrictions, or demand spikes (especially from EV and battery sectors) can inflate costs | High |
Know thy Competitors
Competitive landscape highlights only certain players
Complete list available upon request
Executive Watchlist
| Priority | Immediate Action |
|---|---|
| Diversify Precursor Sourcing | Run feasibility study on non-China lithium and aluminum-based precursor suppliers |
| Safety Compliance Alignment | Initiate facility-wide audit against updated REACH/OSHA pyrophoric material standards |
| Downstream Integration Exploration | Launch OEM/end-user feedback loop to assess custom LAH formulations for fuel cells |
For the Boardroom
To stay ahead in the evolving industry, the board must prioritize dual-track investments: first, in de-risking supply through diversification beyond China for key lithium and aluminum inputs, and second, in compliance and safety innovation to meet tightening global regulatory standards on reactive chemicals.
This intelligence reveals a structural industry shift driven by fuel cell adoption, next-generation battery R&D, and geopolitical volatility, which requires recalibrating the roadmap toward secure sourcing, regulatory alignment, and differentiated formulations tailored for high-growth sectors such as automotive and aerospace. Near-term moves should include initiating strategic sourcing alliances, accelerating the development of pilot-scale custom LAH variants, and establishing a proactive regulatory affairs function.
Segment-wise Analysis
By Purity
The 99% purity segment is expected to register a 63.0% share in 2025. The 99% pure lithium aluminum hydride (LAH) is commonly used due to its high reactivity, reliability, and suitability for critical, high-accuracy applications. The ultra-pure variant is essential in sectors such as pharmaceuticals, semiconductors, and advanced energy systems, where even minute impurities can cause product inefficiencies or malfunction.
In drug manufacturing, for example, rigorous regulatory requirements (such as cGMP and ICH guidelines) require the use of high-purity raw materials. Likewise, in electronics and battery manufacturing, impurities can severely impair the performance of sensitive components.
By Preparation Method
The high-pressure synthesis segment is expected to register a 68.0% share in 2025. The High-Pressure Synthesis process is commonly employed to produce LAH because it is efficient, scalable, and yields higher-quality products. It involves the reaction of aluminum and lithium metals in a controlled high-pressure environment, commonly under a hydrogen or nitrogen atmosphere.
It preserves the creation of very pure and stable LAH, which is essential for applications with consistent performance requirements, such as pharmaceuticals, electronics, and specialty chemicals. In comparison to other techniques, such as the Schlesinger method, high-pressure synthesis offers finer control over reaction conditions, reduces contamination, and enables bulk production with fewer operational risks.
By End-Use Industry
The pharmaceutical industry segment is expected to register a 38.0% share in 2025. The pharmaceutical industry is the most significant end-use industry for lithium aluminum hydride (LAH) as it plays a vital role as a very powerful reducing agent in the synthesis of sophisticated drug intermediates and active pharmaceutical ingredients (APIs). LAH finds special utility in reducing esters, acids, and ketones to alcohols; hence, it is a "must-have" in the production of many antivirals, antimalarials, and antiretroviral drugs.
In addition, studies have proven that LAH inhibits enzymes like DPP-IV, which are associated with metabolic disorders, and even exhibits antiviral activity by interfering with viral replication, enhancing its prospects in drug discovery for diseases such as HIV and malaria.
Country-wise Analysis
| Countries | CAGR |
|---|---|
| USA | 6.1% |
| UK | 4.8% |
| France | 4.5% |
| Germany | 5.0% |
| Italy | 4.2% |
| South Korea | 5.3% |
| Japan | 5.1% |
| China | 5.3% |
USA
The US industry is expected to grow at a CAGR of 6.1% during the forecast period. The country's dominance in the automotive sector, specifically the widespread adoption of electric vehicles (EVs), drives this growth. LAH is crucial in the production of lightweight materials essential for enhancing EV efficiency and range.
The chemical and pharmaceutical industries in the USA are also growing, utilizing LAH as a powerful reducing agent in numerous manufacturing processes. The country's focus on hydrogen fuel cell technology also increases LAH demand, as it is used in hydrogen storage solutions. All these factors together make the USA a large and rapidly growing market for LAH over the next decade.
UK
The UK’s sales are expected to register at a CAGR of 4.8% from 2025 to 2035. The growth here is supported by the UK's robust pharmaceutical industry, which uses LAH comprehensively in the production and manufacture of drugs.
Support from the government for green energy programs and a shift towards hydrogen-based energy systems are also among the factors augmenting the demand for LAH, especially in the form of hydrogen storage systems. Additionally, the UK's emphasis on chemical R&D promotes innovation, leading to wider adoption of LAH.
France
The industry is projected to grow at a compound annual growth rate (CAGR) of 4.5% between 2025 and 2035. The nation's focus on renewable energy and hydrogen fuel technology developments is a major stimulus for this growth. The strong pharmaceutical and chemical industries in France also play a major role in driving demand for LAH, utilizing it as a reducing agent in numerous synthesis reactions.
The government's efforts to reduce carbon emissions and promote sustainable industrial practices also favor the growing adoption of LAH, particularly in energy storage and fuel cells. Industry growth, however, can be impeded by regulation limitations and the threat of competing technologies.
Germany
In Germany, the landscape is anticipated to achieve a CAGR of 5.0% from 2025 to 2035. The nation's dominance in automotive technology, particularly in the development of electric and hydrogen fuel cell vehicles, significantly drives LAH demand. The robust chemical industry in Germany is also dependent upon LAH for numerous synthesis steps.
The government's commitment to sustainable energy solutions, combined with substantial investments in hydrogen infrastructure, further bolsters growth. Collaborations between research institutions and industries in Germany foster innovation, leading to new applications of LAH and enhanced production techniques. These combined factors position Germany as a key player in the European LAH industry.
Italy
In Italy, the industry is projected to grow at a 4.2% compound annual growth rate (CAGR) during the forecast period. The nation's chemical and pharmaceutical sectors are significant consumers of LAH, utilizing it in various manufacturing processes. Italy's emphasis on renewable energy projects and the slow acceptance of hydrogen-based technologies drive the consistent demand for LAH.
Moreover, economic volatility and the rate of technological progress in the nation temper growth. Additionally, consistent investment in research and development activities will drive growth by identifying new uses for LAH.
South Korea
In South Korea, the sector is projected to expand at a compound annual growth rate (CAGR) of 5.3% between 2025 and 2035. South Korea's strong focus on innovation and technology, especially in the automotive and electronics industries, influences LAH demand. South Korea's investments in hydrogen fuel cell technology and the development of hydrogen infrastructure further enhance industry opportunities.
The favorable policies of the government and efforts to encourage green energy solutions are closely aligned with the increasing adoption of LAH, particularly in energy storage. Academic institutions and industries collaborate to create research and development, leading to new applications and improved production processes for LAH.
Japan
The LAH industry in Japan is projected to grow at a compound annual growth rate (CAGR) of 5.1% during the forecast period. The nation's sophisticated automotive sector, which is oriented toward the hydrogen fuel cell, plays a major role in driving LAH demand. Japan's dedication to hydrogen as a clean energy source and heavy investments in hydrogen infrastructure also support growth.
The chemical and pharmaceutical sectors in Japan also use LAH in different synthesis processes. Government policies supporting sustainable energy alternatives and partnerships between industries and research organizations spur innovation, resulting in new uses and improved production methods for LAH.
China
The industry in China is expected to expand at a compound annual growth rate (CAGR) of 5.3% from 2025 to 2035, driven by the country's substantial industrial base and strong demand for domestic self-sufficiency in specialty chemicals and advanced materials.
With China being the world's largest manufacturing center, it plays a crucial role in producing electronic components, chemicals, and pharmaceutical intermediates for all major end-use applications, including LAH. The pharmaceutical and electronics sectors are growing strongly, driven by government support and export demand, thereby driving the adoption of LAH.
Market Share Analysis
Oakwood Chemical: 2.5%
United States-based Oakwood Chemical is a fine chemicals and reagents company that offers LAH for organic synthesis. It caters to R&D laboratories, pharma manufacturers, and CROs in North America and parts of Europe. Although its size is smaller than that of multinational giants, its consistency and product purity give it a competitive advantage in specialty research industries.
Loba Chemie: 2%
Loba Chemie, India-based, provides lab-grade LAH to academic and industrial customers across South Asia, the Middle East, and Africa. Its focus on cost-effectiveness, inventory availability, and regional distribution support provides it with a significant presence in cost-sensitive but expanding areas such as India, Bangladesh, and the UAE.
Alfa Aesar :1.8%
Though Alfa Aesar is part of Thermo Fisher, it still has unique industry activity in selling high-purity and research-grade LAH in Europe and Asia. Its catalog-style, on-demand distribution business model attracts universities, government laboratories, and boutique pharmaceutical firms.
Spectrum Chemical Mfg. Corp.:1.5%
Spectrum Chemical is an American supplier of analytical and laboratory-scale chemicals, including LAH. Its customers are laboratories, government institutions, and pharmaceutical researchers. Its broad product documentation and certifications also serve to meet compliance requirements in regulated settings.
Acros Organics (Fisher Scientific brand) :1.3%
Acros Organics, which has operations throughout Europe and North America, distributes smaller-quantity LAH for research purposes. It is to be found in universities and contract synthesis companies. Although its share of the industry is modest, it has an important impact on non-industrial demand.
MP Biomedicals:1%
MP Biomedicals supports both life sciences and fine chemical segments, with a small LAH offering found mainly used in molecular synthesis and laboratory procedures. Its focus in scientific applications makes its distribution small but powerful in some high-tech areas.
Other Key Players
- Albemarle Corporation
- American Elements
- Cymit Química S.L.
- Merck KGaA
- Mitsubishi Chemicals
- SRL Pvt. Ltd.
- Thermo Fisher Scientific
- Tokyo Chemical Industry Co., Ltd.
- Other Prominent Players
Segmentation
By Purity:
- 95%
- 97%
- 99%
By Preparation Method:
- Schlesinger Method
- High-pressure Synthesis Method
By End-Use Industry:
- Electronics
- Chemical
- Energy & Power
- Pharmaceutical
- Others
By Region:
- North America
- Latin America
- Europe
- Asia Pacific
- Middle East and Africa (MEA)
Table of Content
- Market - Executive Summary
- Market Overview
- Market Background and Foundation Data
- Global Demand (Tons) Analysis and Forecast
- Global Market - Pricing Analysis
- Global Market Value (USD million) Analysis and Forecast
- Global Market Analysis and Forecast, By Purity
- 95%
- 97%
- 99%
- Global Market Analysis and Forecast, By Preparation Method
- Schlesinger Method
- High-pressure Synthesis Method
- Global Market Analysis and Forecast, By End-Use Industry
- Electronics
- Chemical
- Energy & Power
- Pharmaceutical
- Others
- Global Market Analysis and Forecast, By Region
- North America
- Latin America
- Europe
- East Asia
- South Asia & Oceania
- Middle East & Africa
- North America Market Analysis and Forecast
- Latin America Market Analysis and Forecast
- Europe Market Analysis and Forecast
- East Asia Market Analysis and Forecast
- South Asia & Oceania Market Analysis and Forecast
- Middle East & Africa Market Analysis and Forecast
- Country-level Market Analysis and Forecast
- Market Structure Analysis
- Competition Analysis
- Albemarle Corporation
- American Elements
- Cymit Química S.L.
- Merck KGaA
- Mitsubishi Chemicals
- SRL Pvt. Ltd.
- Thermo Fisher Scientific
- Tokyo Chemical Industry Co., Ltd.
- Other Prominent Players
- Assumptions & Acronyms Use
- Research Methodology
Don't Need a Global Report?
save 40%! on Country & Region specific reports
- FAQs -
How big is the lithium aluminum hydride market?
The industry is anticipated to reach USD 190.1 million in 2025.
What is the outlook on lithium aluminum hydride sales?
The industry is predicted to reach a size of USD 313.3 million by 2035.
Who are the key lithium aluminum hydride companies?
Prominent players include Albemarle Corporation, American Elements, Cymit Química S.L., Merck KGaA, Mitsubishi Chemicals, SRL Pvt. Ltd., Thermo Fisher Scientific, Tokyo Chemical Industry Co., Ltd., and other prominent players that dominate the lithium aluminum hydride market with their strong global presence, diversified portfolios, and advanced synthesis capabilities.
Which is the major end-use industry of lithium aluminum hydride?
The pharmaceutical industry is the major end user.
Which country is likely to witness the fastest growth in the lithium aluminum hydride market?
China, set to grow at 5.3% CAGR during the forecast period, is poised for the fastest growth.