Healthcare Contract Research Outsourcing Market
Healthcare Contract Research Outsourcing Market Trend Analysis Based on Service, Therapeutic Area, End-User, and Region (2025 to 2035)
Analysis of Healthcare Contract Research Outsourcing Market Covering 30+ Countries Including Analysis of US, Canada, UK, Germany, France, Nordics, GCC countries, Japan, Korea and many more
Healthcare Contract Research Outsourcing Market Outlook (2025 to 2035)
The healthcare contract research outsourcing market is valued at USD 53.1 billion in 2025. As per Fact.MR’s analysis, the healthcare contract research outsourcing industry will grow at a CAGR of 6.6% and reach USD 100.93 billion by 2035.
In 2024, the healthcare contract research outsourcing industry saw consistent growth, fueled by rising regulatory complexities and the increasing use of CROs for drug development and management of clinical trials. The need for precision medicine and personalized treatment strategies accelerated the move toward outsourcing, as pharma companies looked for specialized skills and cost-effective solutions.
Looking forward to 2025, the sector is on course for greater growth as pharmaceutical and biotech companies ramp up their emphasis on innovation while minimizing operational expense. The increasing weight of regulatory approvals and the necessity for quicker drug development pipelines are likely to propel greater use of contract research services.
| Metric | Value |
|---|---|
| Industry Value (2025E) | USD 53.1 billion |
| Industry Value (2035F) | USD 100.93 billion |
| CAGR (2025 to 2035) | 6.6% |
Don't Need a Global Report?
save 40%! on Country & Region specific reports
Market Analysis
The medical contract research outsourcing industry is a rising trend on the back of growing regulatory needs and the enhancing requirement for affordable drug development. Pharmaceutical and biotechnology companies are utilizing CROs to advance clinical trials at faster rates as well as weather tough compliance stipulations. With increasing demand helping traditional CROs, their less technologically integrated or short-staffed counterparts might miss the pace the industry is increasingly demanding.
Top 3 Strategic Imperatives for Stakeholders
Tap Advanced Technologies
Combine AI, real-world evidence, and automation to speed clinical trials, improve regulatory compliance, and reduce drug development time.
Maximize Regulatory Navigation
Deepen global regulatory capabilities to achieve smooth drug approvals and increase presence in high-growth sectors.
Power Strategic Collaborations
Form alliances and make selective acquisitions to extend service capabilities, broaden sector coverage, and remain competitive.
More Insights, Lesser Cost (-50% off)
Insights on import/export production,
pricing analysis, and more – Only @ Fact.MR
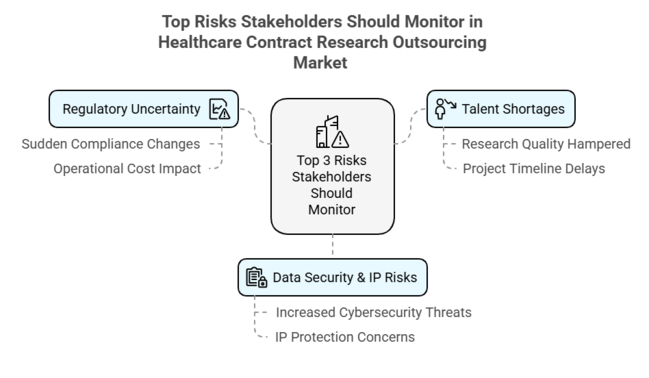
Top 3 Risks Stakeholders Should Monitor
| Risk | Probability - Impact |
|---|---|
| Regulatory Uncertainty - Sudden changes in worldwide compliance mandates can slow approvals and raise the costs of operations. | High - High |
| Talent Shortages - Shortage of available skilled professionals can hamper research quality and project timelines. | Medium - High |
| Data Security & IP Risks - Increased cybersecurity threats and intellectual property protection concerns are a major challenge. | High - Medium |
1-Year Executive Watchlist
| Priority | Immediate Action |
|---|---|
| Regulatory Agility | Implement a rapid-response compliance team to anticipate, adapt, and implement emerging global regulations. |
| Talent Fortification | Initiate rigorous talent search and specialized training programs to fill skill gaps. |
| Data Sovereignty & IP Defense | Strengthen cybersecurity systems and implement tight IP protection measures to help counteract risks. |
Know thy Competitors
Competitive landscape highlights only certain players
Complete list available upon request
For the Boardroom
To stay ahead, companies must embrace cutting-edge technologies, strengthen regulatory capabilities, and build high-impact collaborations to drive drug development and simplify clinical trials.
Enhancing AI-powered research, growing global compliance capabilities, and attracting top talent will be essential to sustaining a competitive advantage. The changing healthcare environment requires an active, innovation-led strategy-one that focuses on speed, accuracy, and strategic partnerships to unlock new growth opportunities and improve operational resilience.
Segment-Wise Analysis
By Service
The healthcare contract research outsourcing industry is changing fast, with clinical trial services at the forefront. Increasing drug development complexity and tight approval timelines are driving demand for outsourced trials, promoting efficiency and cost savings.
Regulatory services are also building momentum as companies deal with increasingly dynamic compliance environments, facilitating quicker approvals and sector access. Clinical data management and biometrics are becoming essential, and AI-based analytics is being used to improve data integrity and encourage reporting.
Medical writing is seeing steady growth, with greater focus on accurate documentation for regulatory filings.
Pharmacovigilance is seeing robust growth, as international safety standards become more stringent and post-market surveillance becomes essential. Site management protocol services are changing to maximize trial execution, enhancing patient recruitment and retention. The service ecosystem is developing at a vibrant rate, with the segment expected to expand at a whopping CAGR of 7.2% between 2025 and 2035.
By Therapeutic Area
Oncology and hematology continue to be leaders in healthcare contract research outsourcing, with the rise of targeted therapies and precision medicine fueling collaborations with CROs. Research in central nervous system (CNS) is growing as demand for new treatments in neurodegenerative and psychiatric diseases picks up speed.
Cardiovascular and metabolic disease continues to create a lot of outsourcing activity, as lifestyle diseases increase and drug pipelines grow. Respiratory research is gaining momentum, fueled by growing interest in chronic diseases like asthma and COPD. Infectious disease research is experiencing consistent outsourcing, powered by worldwide pandemic preparedness and antibiotic resistance issues.
Immunology continues to be a robust space for CRO involvement, especially in autoimmune disease research and biologics development. Rare diseases are gaining increased investments, with regulatory incentives and orphan drug approvals fueling demand for outsourcing. Medical device-related research is seeing strong growth, with changing safety standards and advancements in wearables driving demand for CRO services.
Other therapy segments, such as dermatology and ophthalmology, remain growing as models of outsourcing demonstrate their effectiveness. This segment is expected to increase at a CAGR of 6.9% from 2025 through 2035.
By End User
Pharma firms continue to be the biggest backers of contract research outsourcing, taking advantage of CRO know-how to speed drug development and reduce regulatory burdens. Biotech companies are increasingly collaborating with CROs in order to leverage cutting-edge research capacity, especially in gene therapy and biologics. Medical device manufacturers continue to increase their use of outsourcing, propelled by increasing regulatory attention and the need to speed clinical verification.
Government institutions and academic institutions are increasingly contributing, outsourcing research functions to close gaps in resources and stimulate innovation. The total demand for end user outsourcing will continue to rise steadily, with the segment likely to post a CAGR of 6.8% between 2025 and 2035.
Country-wise Insights
United States
The United States leads the contract research outsourcing industry for healthcare, fueled by the presence of strong pharmaceutical and biotech industries. The regulatory environment of the country is transforming with the FDA simplifying approval processes for new therapies, leading to higher demand for expertise-driven CRO services.
Clinical trial solutions based on artificial intelligence and decentralized trials are transforming research models, enhancing patient enrollment and data management. Strategic partnerships between CROs and big pharma companies are promoting fast drug development, while an increase in oncology and rare disease research is driving demand for outsourcing.
Fact.MR opines that the CAGR of the U.S. healthcare contract research outsourcing sector will be 6.9% from 2025 to 2035.
India
India is becoming one of the major centers for healthcare contract research outsourcing, powered by a pool of skilled professionals, low-cost operations, and a favorable regulatory environment. The nation is seeing an increase in early-phase clinical trials, fueled by its pool of diverse patients and rising ethical compliance standards.
Digitization of clinical research, such as e-clinical platforms and AI-enabled patient monitoring, is enhancing trial efficiency. The Indian government initiative towards developing research infrastructure is improving the country's global competitiveness.
Growing biosimilar and vaccine research outsourcing further enhances India's position in the industry.
Fact.MR forecasts that the CAGR of India’s healthcare contract research outsourcing sector will be 7.4% from 2025 to 2035.
China
China's contract research outsourcing healthcare industry is expanding rapidly due to its developing biopharmaceutical industry and regulatory reforms that promote innovation. The nation is focusing on fast-track approvals, which make it a hub for early-phase trials.
Increased government spending on R&D and the incorporation of AI-based clinical trial platforms are enhancing trial efficiency and data integrity. The aggressive drive for novel drug commercialization and expanding roles for multinational CROs in China are driving the growth.
Increasing China's capability in cell and gene therapies is also further enhancing its competitiveness on a global basis.
Fact.MR projects that the CAGR of China’s healthcare contract research outsourcing sector will be 7.2% from 2025 to 2035.
United Kingdom
The United Kingdom remains a strategic center for the outsourcing industry of healthcare contract research due to its established clinical research environment. Having top-notch academic institutions and research hospitals promotes innovation in areas such as oncology, rare diseases, and gene therapy.
Post-Brexit regulatory harmonization with international standards is enhancing global research collaborations and introducing more outsourcing opportunities. The UK's high skill base in pharmacovigilance and post-industry surveillance is also boosting its CRO sector.
Fact.MR is of the opinion that the CAGR of the UK’s healthcare contract research outsourcing sector will be 6.8% from 2025 to 2035.
Germany
Germany continues to be a health contract research outsourcing powerhouse due to its strong regulatory environment and sophisticated medical infrastructure. Germany is a leader in early-phase drug research, utilizing AI and automation to make trial processes efficient.
Germany's knowledge of biosimilars and biologics research is drawing multinational investment from pharmaceutical companies, driving demand for CROs. The emphasis placed on data protection and regulation is bolstering the reputation of the outsourcing industry.
The widespread uptake of decentralized clinical trials and wearable technology is also enhancing efficiency in research methods.
Fact.MR forecasts that the CAGR of Germany’s healthcare contract research outsourcing sector will be 6.7% from 2025 to 2035.
South Korea
South Korea is gaining momentum in outsourcing healthcare contract research because of its emerging biotechnology sector and pro-government policies. South Korea is at the forefront of cell and gene therapy clinical trials, drawing international pharmaceutical partnerships. The use of AI-powered trial monitoring and big data analysis is making regulatory processes more efficient.
South Korea's robust clinical research environment and electronic health adoption are also further advancing its CRO scene. The rising need for biosimilars and innovative formulations is further widening the realm of outsourced research activities.
Fact.MR opines that the CAGR of South Korea’s healthcare contract research outsourcing sector will be 7.1% from 2025 to 2035.
Japan
Japan's contract research outsourcing industry is progressively advancing with the support of its preeminence in precision medicine and regenerative therapies. Japan's regulatory overhaul, such as rapid approval paths for new drugs, is accelerating CRO activity. The global presence of giant pharma and cutting-edge research institutions is compelling partnerships in clinical trials and data handling.
The increasing need for real-world evidence studies and outsourcing of pharmacovigilance is further boosting the industry. The increased emphasis on rare diseases and new biologics is broadening research collaborations.
Fact.MR projects that the CAGR of Japan’s healthcare contract research outsourcing sector will be 6.9% from 2025 to 2035.
France
France is solidifying its position in contract research outsourcing in the healthcare space with its robust pharmaceutical industry and high rates of clinical trial participation. The government initiative of promoting medical research innovation is enhancing partnership between CROs and biotech startups.
Growing investments in AI-enabled clinical trial solutions are enhancing efficiency and patient recruitment. France's immunotherapy and vaccine research expertise is fueling robust outsourcing expansion. Decentralized trials and digital health technologies are simplifying regulatory approvals.
The real-world data applications and the focus on pharmacovigilance in the country are further augmenting CRO collaborations.
Fact.MR forecasts that the CAGR of France’s healthcare contract research outsourcing sector will be 6.7% from 2025 to 2035.
Italy
Italy's healthcare contract research outsourcing industry is growing, driven by its expanding biotechnology sector and government-sponsored clinical research programs. The nation's established leadership in biosimilar and vaccine development is drawing international CRO partnerships. Widespread adoption of digital trial platforms and patient monitoring is increasing efficiency.
Italy's adoption of AI-based clinical trial analytics is increasingly enhancing study performance. The emphasis on gene therapy and rare disease research is also making the country's CRO sector more robust.
Fact.MR is of the opinion that the CAGR of Italy’s healthcare contract research outsourcing sector will be 6.6% from 2025 to 2035.
Australia & New Zealand
Australia and New Zealand are solidifying their position in healthcare contract research outsourcing via regulatory flexibility and efficient trial approval procedures. The region's high-quality clinical research infrastructure and patient diversity render it an outsourcing destination of choice. Government incentives for biotech innovation and medical research are driving industry growth.
Growing partnerships among local CROs and multinational pharmaceutical companies are fueling drug development innovations. Growing requirements for precision oncology trials and biologics research are also speeding up outsourcing activities.
Fact.MR opines that the CAGR of Australia & New Zealand’s healthcare contract research outsourcing sector will be 6.8% from 2025 to 2035.
Fact.MR Survey Results: Healthcare Contract Research Outsourcing Industry Dynamics Based on Stakeholder Perspectives
(Surveyed Q4 2024, n=500 stakeholder participants evenly distributed across pharmaceutical companies, biotechnology firms, CRO executives, and regulatory professionals in the US, Western Europe, Japan, China, India, and South Korea.)
Priorities of Stakeholders
- Regulatory Compliance & Data Integrity: 85% of stakeholders cited adherence to changing regulatory environments (FDA, EMA, PMDA) and data protection as a "critical" priority.
- Cost-Effective Trial Management: 78% highlighted cost reduction in drug development through effective outsourcing models.
Regional Variance:
- US: 69% highlighted decentralized clinical trials to facilitate patient recruitment, as opposed to 45% in Japan.
- Western Europe: 81% mentioned sustainability (eco-friendly trial sites, lower carbon footprint) as an important consideration, compared to 50% in China.
- India & China: 67% emphasized quick patient enrollment capacity because of their large and diverse populations, as opposed to 34% in South Korea.
Adoption of Advanced Technologies
High Variance:
- US: 60% of pharma companies embraced AI-based trial analytics for quicker drug approval.
- Western Europe: 52% incorporated real-world evidence (RWE) data into clinical trial plans, spearheaded by Germany (65%).
- China: 48% applied blockchain for protecting clinical data, standing in stark contrast with merely 22% in Japan, due to ongoing fears of excessive costs.
- India & South Korea: 39% invested in cloud-based trial management systems to share data with ease.
Convergent and Divergent Views on ROI:
- 73% of US stakeholders found AI-powered drug development "worth the investment," while 40% in Japan still used the conventional clinical trial models.
Most Popular Outsourcing Models
Consensus:
- Full-Service CROs: Chosen by 68% of stakeholders across the world owing to end-to-end efficiency.
Regional Variance:
- Western Europe: 55% chose functional service providers (FSPs) as opposed to full-service models on account of increased budget control.
- US: 72% used hybrid outsourcing (combination of in-house and outsourced research), versus only 38% in South Korea.
- China & India: 49% were inclined towards offshore preclinical outsourcing for cost savings and speed.
Price Sensitivity & Budget Constraints
Shared Challenges:
- 87% mentioned increasing costs of clinical trials (28% YoY increase) as a key concern.
Regional Variations:
- US & Western Europe: 65% would pay a 20% premium for AI-based CRO services.
- Japan & South Korea: 73% asked for cheaper models of outsourcing (<$10 million per trial) and preferred local CROs.
- China & India: 46% opted for cost-flexible engagement models (e.g., milestone-based pricing), compared to just 20% in the US.
Pain Points in the Value Chain
Manufacturers & CROs:
- US: 57% mentioned patient recruitment and trial retention bottlenecks.
- Western Europe: 50% wrestled with intricate compliance procedures (e.g., GDPR in clinical data sharing).
- India & China: 63% complained of high employee turnover in CROs, affecting project continuity.
Pharmaceutical & Biotech Firms:
- US: 71% mentioned delays in regulatory approval timelines as a major challenge.
- Western Europe: 45% mentioned inefficiencies in data standardization among CROs.
- Japan: 58% mentioned limited experience in emerging therapeutic areas like cell and gene therapy.
Priorities for Future Investments
Alignment:
- 75% of all CROs globally intend to invest in artificial intelligence-based patient monitoring technologies.
Divergence:
- US: 63% invested in digitalized clinical trial workflows aimed at minimizing inefficiencies.
- Western Europe: 58% emphasized sustainability practices, such as green trial facilities and paper-free data handling.
- China & India: 52% made investments in capacity expansion of early-phase trial abilities to win overseas clients.
Regulatory Impacts
- US: 67% of stakeholders considered FDA's changing trial regulations (e.g., decentralized trial guidance) a long-term industry driver.
- Western Europe: 80% expected the EU Clinical Trial Regulation (2022) to spur demand for specialist CRO collaborations.
- Japan & South Korea: Just 35% saw regulations having a major influence on outsourcing trends as a result of delayed enforcement.
Conclusion: Variance vs. Consensus
High Consensus:
- Cost pressures, regulatory compliance, and growing AI-based clinical trials are transforming the global CRO industry.
Key Differences:
- US: Significant automation investments vs. Japan: Cost-controlled outsourcing preference.
- Western Europe: Pioneers in sustainability strategies vs. Asia: Cost-flexible trial designs.
Strategic Insight:
- A one-size-fits-all solution will not provide success. Customization by region-like hybrid outsourcing in America, cost-driven engagement in Asia, and environment-friendly experiments in Europe-will shape competitive edge.
Government Regulations
| Country | Regulatory Impact & Certifications |
|---|---|
| United States | FDA requires GCP adherence, IND/NDA approval, and 21 CFR Part 11 compliance. Decentralized trials are becoming regulatory compliant. |
| India | CDSCO regulates New Drugs and Clinical Trial Rules, 2019. CROs require Form 11 certification and ethics committee approval. |
| China | NMPA strictly requires GCP adherence. Foreign CROs have to be teamed up with local companies for trial approval. |
| United Kingdom | MHRA works independently post-Brexit. CROs require UK GCP adherence and Clinical Trial Authorisation (CTA). |
| Germany | BfArM is subject to EMA guidelines, necessitating EU CTR 536/2014 and ISO 14155 certification for medical devices. |
| South Korea | MFDS requires Korea GCP (KGCP). Multinational CROs benefit from fast-track approval of trials. |
| Japan | PMDA necessitates Clinical Trials Act compliance with strict post-market surveillance. Domestic data requirements hinder foreign approval. |
| France | ANSM conforms to EU CTR. Public hospital studies require CPP (ethics committee) approval, increasing delays. |
| Italy | AIFA requires EU CTR and GDPR compliance. Ethics committee approvals delay the initiation of trials. |
| Australia-NZ | TGA/Medsafe adhere to ICH-GCP. CTN scheme provides for rapid trial approvals, although data localization policies are becoming stricter. |
Competitive Landscape
The industry for healthcare contract research outsourcing is fairly concentrated with dominant players acquiring substantial industry share.
Industry leaders like ICON Plc, Charles River Laboratories, Syneos Health, and IQVIA Inc. compete based on pricing strategies, technological innovations, strategic alliances, and international expansions.
In 2024, Charles River Laboratories made a deal with Pluristyx Inc. for access to research tools to help hasten therapeutic development. Thermo Fisher Scientific introduced CorEvitas, a Generalized Pustular Psoriasis (GPP) clinical registry, further solidifying its dermatology trial presence. Parexel International Corporation deepened its presence in Japan through a strategic partnership with the Japanese Foundation for Cancer Research, enhancing oncology clinical trial availability.
Sonic Healthcare secured a notable German acquisition through the purchase of Laboratory Group Dr. Kramer and Colleagues (LADR) for €423 million, strengthening its foothold in Europe.
Market Share Analysis
IQVIA - 22.5%
- Dominates the industry with full-service clinical research and real-world evidence solutions.
LabCorp (Covance) - 18.3%
- Dominant in central lab services and drug development support.
Syneos Health - 12.7%
- Integrated clinical and commercial CRO industry leader.
Parexel - 11.2%
- Expert in regulatory consulting and late-phase research.
PRA Health Sciences (ICON plc) - 9.8%
- Recognized for clinical trial management and data solutions.
Charles River Laboratories - 7.5%
- Early-stage research and preclinical services industry leader.
Key Industry Players Include
- IQVIA
- ICON plc
- LabCorp (Covance)
- Syneos Health
- Parexel
- Charles River Laboratories
- PPD (Thermo Fisher Scientific)
- Medpace
- WuXi AppTec
- PRA Health Sciences
- Fortrea
- Catalent
- SGS Life Sciences
- CMIC Group
- EPS Holdings
Segmentation
By Service:
Clinical Trial Services, Regulatory Services, Clinical Data Management & Biometrics, Medical Writing, Pharmacovigilance, Site Management Protocol, Others.
By Therapeutic Area:
Oncology/Hematology, CNS, CV/Metabolic, Respiratory, Infectious Diseases, Immunology, Rare Diseases, Medical Devices, Others.
By End User:
Pharmaceutical Companies, Biotechnology Companies, Medical Device Companies, Academic Institutes & Government Organizations.
By Region:
North America, Latin America, Europe, East Asia, South Asia & Oceania, Middle East and Africa (MEA).
Table of Content
- 1. Global Market - Executive Summary
- 2. Global Market Overview
- 3. Market Risks and Trends Assessment
- 4. Market Background and Foundation Data Points
- 5. Global Market Demand (US$ Mn) Analysis 2020 to 2024 and Forecast, 2025 to 2035
- 6. Global Market Analysis 2020 to 2024 and Forecast 2025 to 2035, By Service
- 6.1. Clinical Trial Services
- 6.2. Regulatory Services
- 6.3. Clinical Data Management & Biometrics
- 6.4. Medical Writing
- 6.5. Pharmacovigilance
- 6.6. Site Management Protocol
- 6.7. Others
- 7. Global Market Analysis 2020 to 2024 and Forecast 2025 to 2035, by Therapeutic Area
- 7.1. Oncology/Hematology
- 7.2. CNS
- 7.3. CV/Metabolic
- 7.4. Respiratory
- 7.5. Infectious Diseases
- 7.6. Immunology
- 7.7. Rare Diseases
- 7.8. Medical Devices
- 7.9. Others
- 8. Global Market Analysis 2020 to 2024 and Forecast 2025 to 2035, by End User
- 8.1. Pharmaceutical Companies
- 8.2. Biotechnology Companies
- 8.3. Medical Device Companies
- 8.4. Academic Institutes & Government Organizations
- 9. Global Market Analysis 2020 to 2024 and Forecast 2025 to 2035, by Region
- 9.1. North America
- 9.2. Latin America
- 9.3. Europe
- 9.4. East Asia
- 9.5. South Asia & Oceania
- 9.6. Middle East and Africa (MEA)
- 10. North America Market Analysis 2020 to 2024 and Forecast 2025 to 2035
- 11. Latin America Market Analysis 2020 to 2024 and Forecast 2025 to 2035
- 12. Europe Market Analysis 2020 to 2024 and Forecast 2025 to 2035
- 13. East Asia Market Analysis 2020 to 2024 and Forecast 2025 to 2035
- 14. South Asia & Oceania Market Analysis 2020 to 2024 and Forecast 2025 to 2035
- 15. Middle East and Africa Market Analysis 2020 to 2024 and Forecast 2025 to 2035
- 16. Market Structure Analysis
- 17. Competition Analysis
- 17.1. IQVIA
- 17.2. ICON plc
- 17.3. LabCorp (Covance)
- 17.4. Syneos Health
- 17.5. Parexel
- 17.6. Charles River Laboratories
- 17.7. PPD (Thermo Fisher Scientific)
- 17.8. Medpace
- 17.9. WuXi AppTec
- 17.10. PRA Health Sciences
- 17.11. Fortrea
- 17.12. Catalent
- 17.13. SGS Life Sciences
- 17.14. CMIC Group
- 17.15. EPS Holdings
- 18. Assumptions and Acronyms Used
- 19. Research Methodology
Don't Need a Global Report?
save 40%! on Country & Region specific reports
List Of Table
More Insights, Lesser Cost (-50% off)
Insights on import/export production,
pricing analysis, and more – Only @ Fact.MR
List Of Figures
Know thy Competitors
Competitive landscape highlights only certain players
Complete list available upon request
- FAQs -
What is driving the growth of healthcare contract research outsourcing?
The upsurge in sophisticated clinical trials, changing regulatory landscapes, and the drive for cost containment are forcing pharmaceutical and biotech companies to tap into expert outsourcing solutions.
What technologies are reshaping healthcare research outsourcing?
Trial optimization through artificial intelligence, real-time monitoring of patients, and cloud-based data aggregation are transforming drug development with greater precision, speed, and regulatory compliance.
Which areas are becoming global centers for outsourced healthcare research?
Asia-Pacific and Eastern Europe are becoming increasingly popular because of cost benefits, regulatory reform, and a qualified workforce, making them key outsourcing locations.
What are the challenges companies encounter in outsourcing clinical research?
Dealing with intricate international regulations, protecting sensitive information, and ensuring quality control over decentralized operations continue to be key challenges in the outsourcing environment.
Why are pharmaceutical behemoths increasing their dependence on contract research organizations?
The need to pursue expedited drug approvals, lower in-house R&D expenses, and leverage specialized expertise is causing a sector-wide trend toward strategic outsourcing collaborations.